The Microbiome modulates immune defense pathways thereby altering host susceptibility to infection.
Our research focuses on the pathogenesis of and host response to Clostridioides difficile, a bacterium that infects the large intestine following perturbation of the intestinal microbiota. C. difficile represents one of the most urgent public health threats in the United States with high recurrence rates following antibiotic treatment, which highlights the need to identify alternative strategies to control this disease.
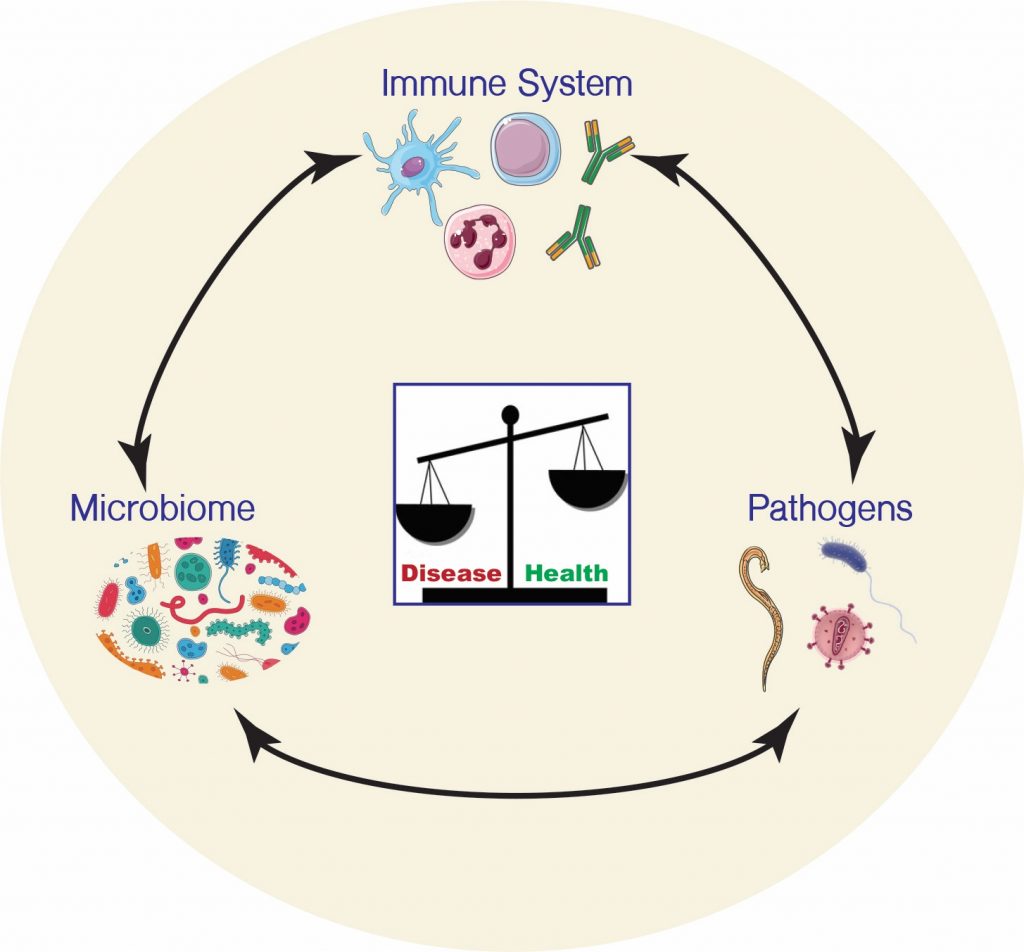
Immune System
The mammalian immune system has evolved multiple defense mechanisms to efficiently protect the host from a myriad of diverse pathogens. Our lab research seeks to unravel these elaborate components of host immunity for the purpose of harnessing the power of the immune system for therapeutic intervention. Specifically, the lab studies cellular innate immunity at mucosal barrier surfaces.
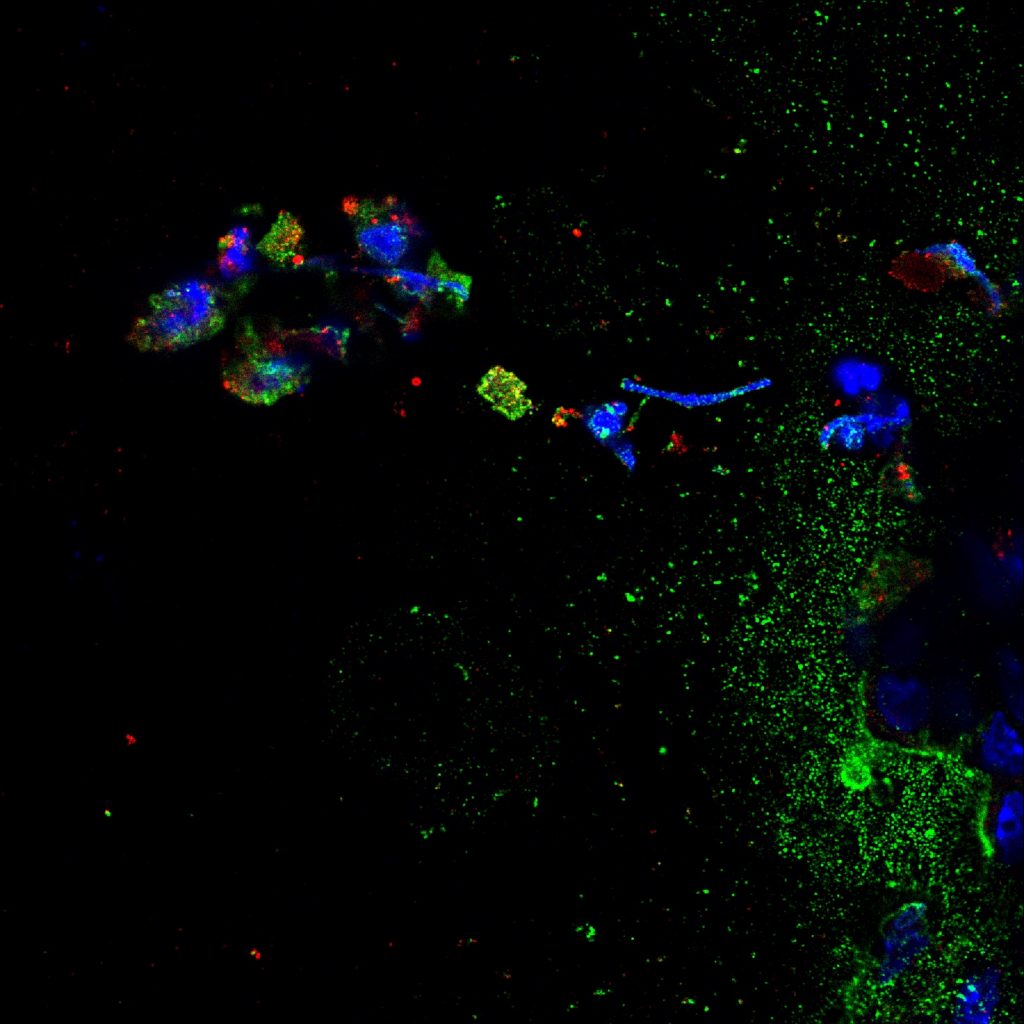
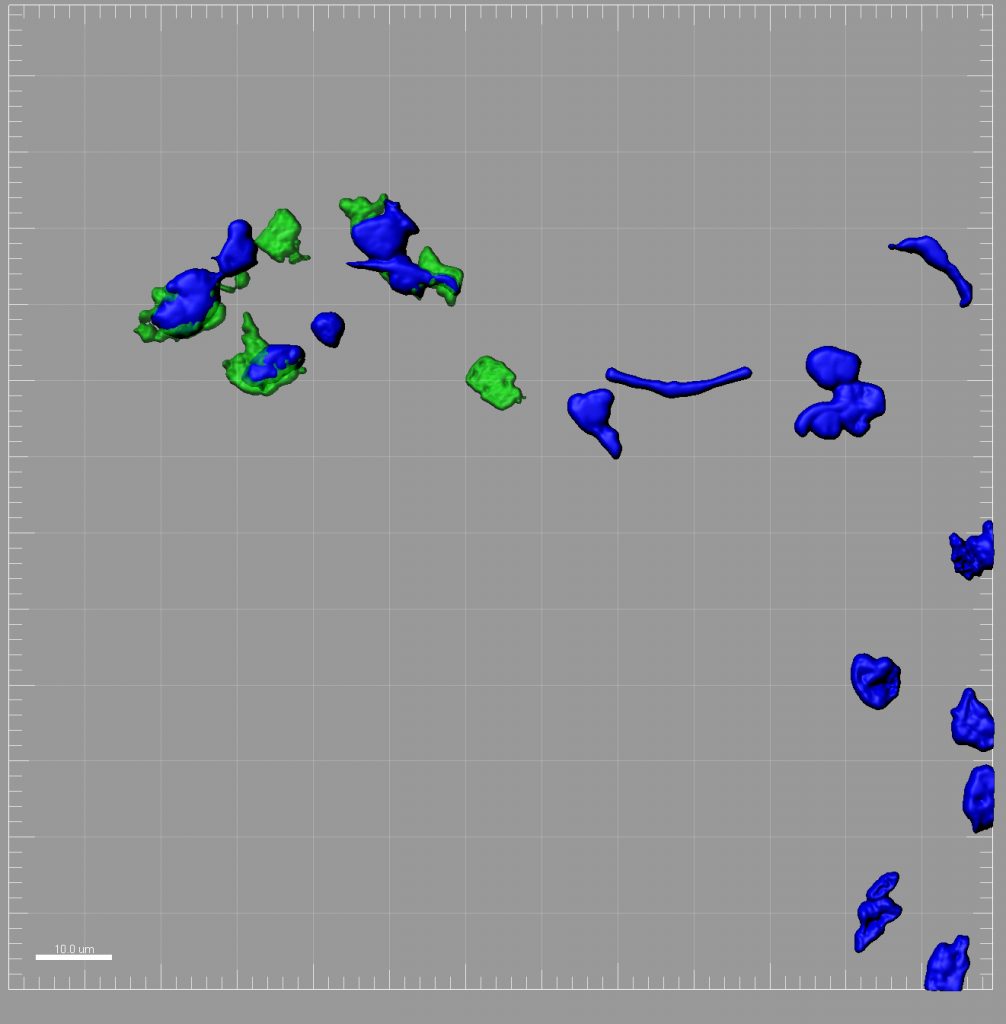
Pathogens
One of our lab’s main research focuses is the pathogenesis of and host response to Clostridioides difficile, a bacterium that infects the large intestine following perturbation of the intestinal microbiome. C. difficile is one of the most urgent public health threats in the United States. Infected patients experience a high rate of recurrence rates following standard antibiotic treatment. This highlights the need to better understand the pathogen and identify alternative strategies to control this disease.
There are four main factors that determine the severity of C. difficile pathogenesis: 1.) The bug itself, (the strain of C. difficile ) 2.) the composition of the Microbiome, 3.) the host’s diet, and 4.) the host’s immune system.
The interactions of these four factors determine severity of disease. In the Abt lab, we take a systematic approach by standardizing three of these factors then modulating the fourth to understand its contribution to disease. Using a murine model of C. difficile infection and human clinical samples, our research team investigates immune-microbiota regulation of C. difficile associated disease.
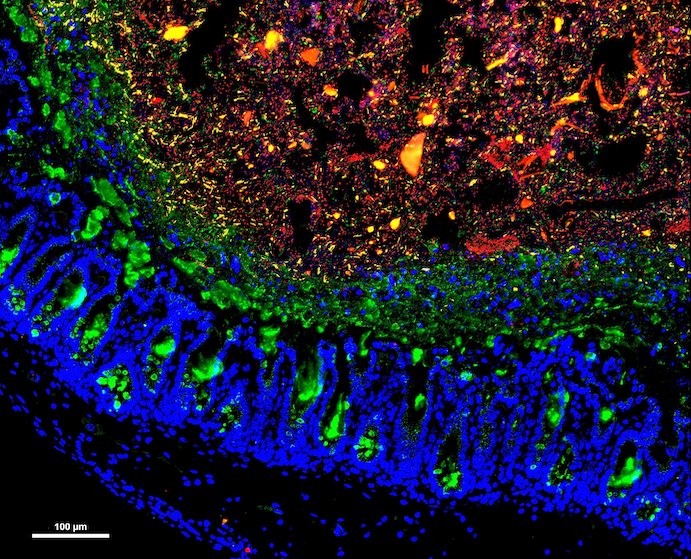
Microbiome
C. difficile is the first disease effectively treated with fecal microbiome transplantation, a microbiome-based therapy. Using microbiome-based therapeutics have been suggested to treat a wide range of maladies from Inflammatory Bowel Disease to Cancer to Neurobiological Disorders. Implementation of this kind of therapy as a viable treatment option is limited due to inadequate understanding of its mechanism of action. Using gnotobiotic mice, high-throughput next-generation DNA sequencing, and targeted metabolomics our research team investigates the underlying mechanisms of how Microbiome therapy successfully treats C. difficile associated disease. These studies seek to reveal insights that are broadly applicable to the treatment of infectious or inflammatory diseases driven by dysbiosis of the microbiome.


